Especially, be aware of that the FDA has expanded the scope on Data Integrity to also now include IT security.
Therefore, as a GMP manufacturer, you must pay careful attention to potential breaches of regulatory requirements on Data Integrity in connection with employees working from home connected to your GMP environment from their private network.
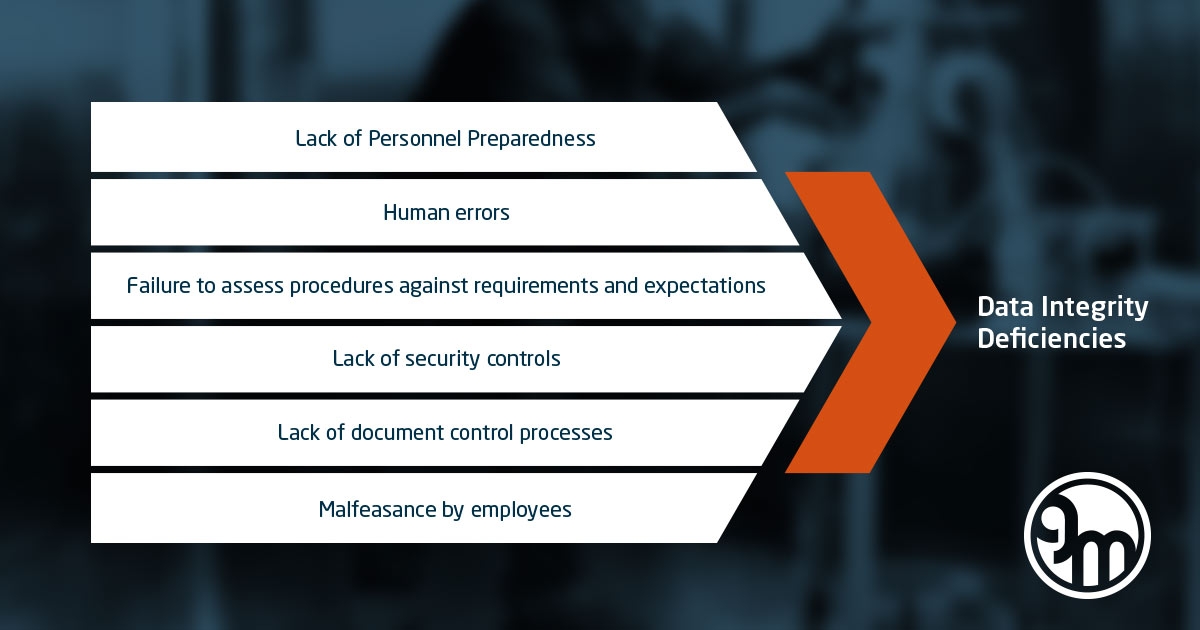
We have put together a portfolio of different input on Data Integrity in general. Please find below 2 video interviews with experts with Data Integrity, incl. a pod cast.
#2 The authorities have gained an increased focus on data integrity
This episode is the second of a series of Podcasts where our CEO Henrik Johanning discusses topics with different Life Science specialists.
In this episode, Henrik Johanning has invited counselor Michael Weng, who is a specialist within Computer System Validation (CSV) and Data Integrity, to a talk where they include:
- Data
- Inspection Readiness
- IT-systems
And why it is so important to map the data flow to be able to make the necessary risk assessment.
The whole talk is in Danish.
Finally, we have published an E-book, which contains among other things 5 basic principles for good GMP Data Control & Data Integrity
Hvis du vil høre mere om hvordan Genau & More kan hjælpe dit firma, så kontakt

Simon Bagger









