This 2nd video (out of 9 in total) focuses on FDA Form 483 observations and Warning Letters, overall statistics for 2019 (and partly 2020) for Drug GMP.
Here you will find, a 9% increase in number of FDA-483's in 2019 compared to 2018. In absolute numbers, 779 FDA-483’s in 2019 as compared to 716 in 2018.
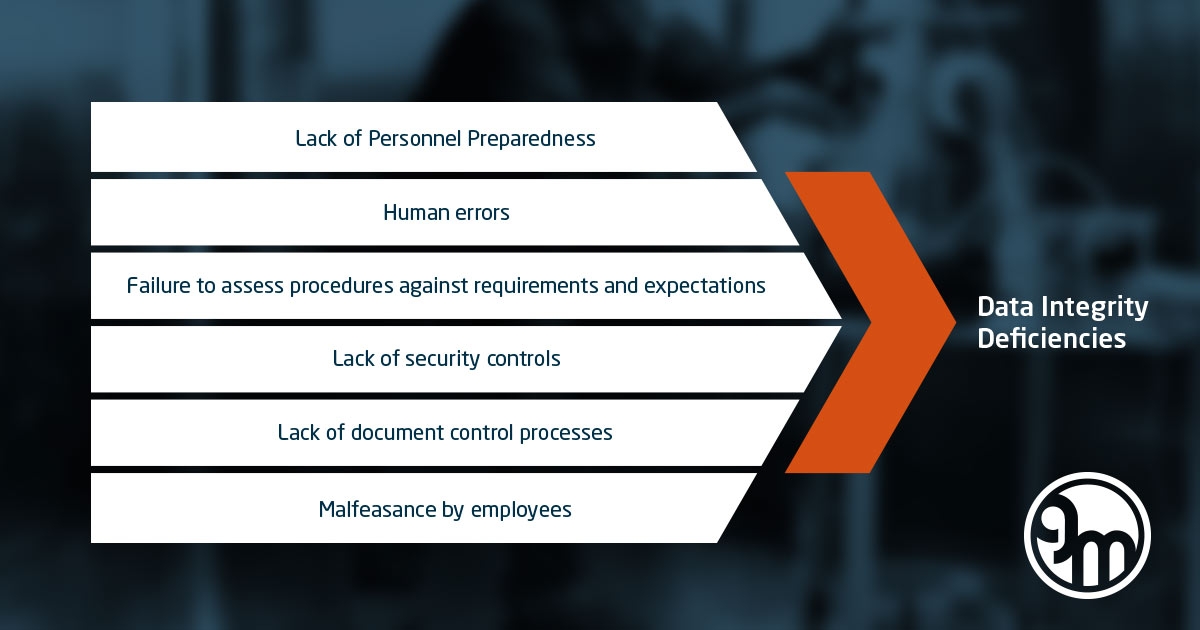
Top 3 issues in FDA-483’s are in 2019 (still) violation of CFR,
• 211.22(d), Quality Assurance Oversight
• 211.192, Investigations
• 211.160(b), Laboratory Control
98 Warning Letters were issued in 2019. That is an 8% increase compared to 2018. As per August 2020, 66 Warning Letters are issued.
Top 3 issues in FDA Warning Letters are,
• Validation
• QA Oversight
• Laboratory Controls
In general, you can access FDA's Inspection Citation database. It covers general citations, e.g. Drug, Device and Part 11 Compliance: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-references/inspection-citation
The video is recorded during the COVID-19 US lock-down by John Lee with mobile recording equipment, and the quality could be better. Focus on the great presentation and content instead.